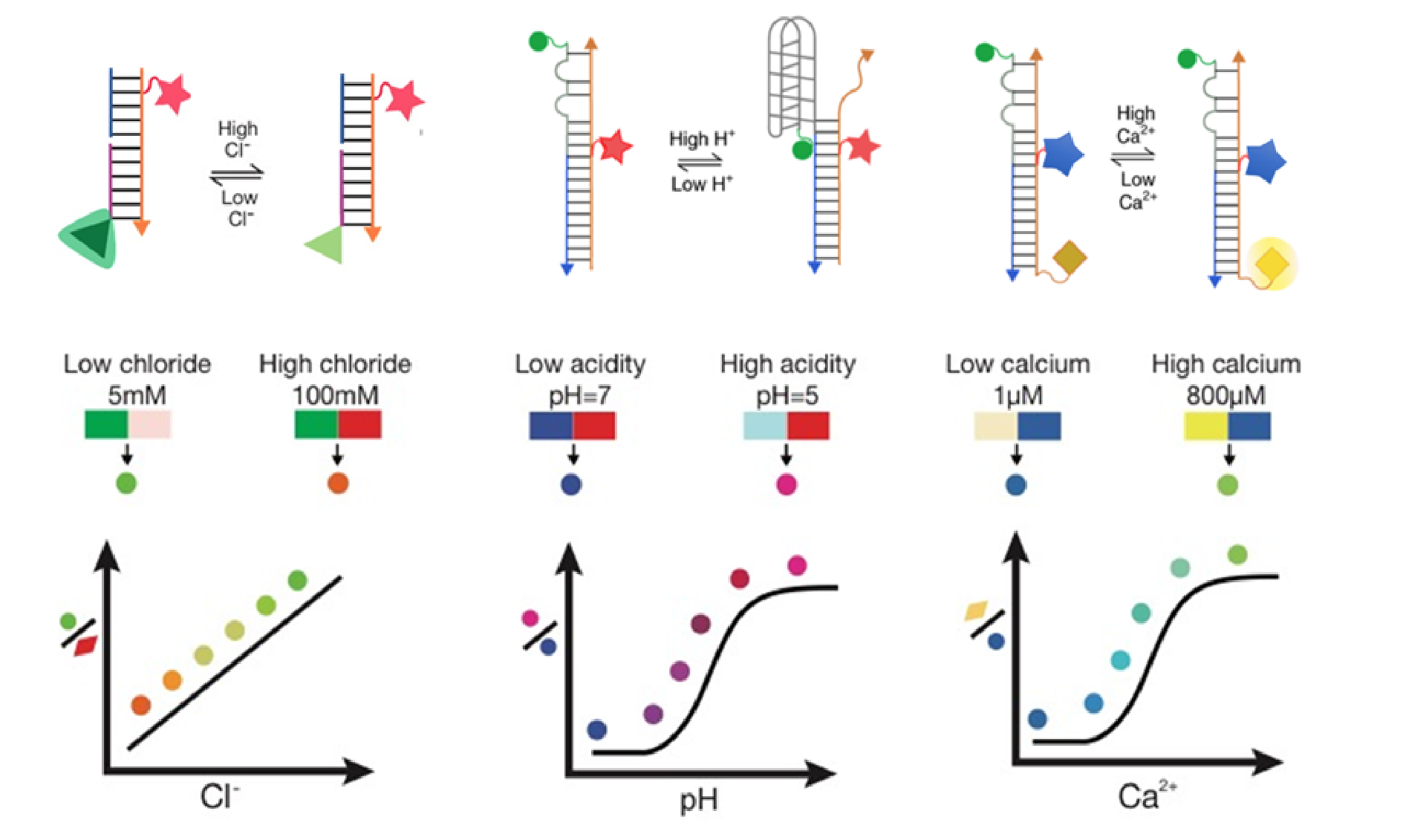
Quantitative, Fluorescent Chemical Sensors
Why develop DNA nanodevices as fluorescent reporters when a range of fluorescent proteins exist? DNA is modular, allowing the integration of independent and interdependent functionalities onto one assembly. By leveraging the 1:1 stoichiometry in DNA duplexes, we can integrate multiple modules with distinct functions in precise stoichiometries onto a single DNA nanodevice. These include (1) a module that acts as fluorescent reporter of a desired analyte (2) a normalizing module for ratiometric quantitation, and (3) a targeting module that localizes the reporter in a specific organelle. We have thus made fluorescent reporters that can quantitatively image ions, reactive species, and enzymatic activity.
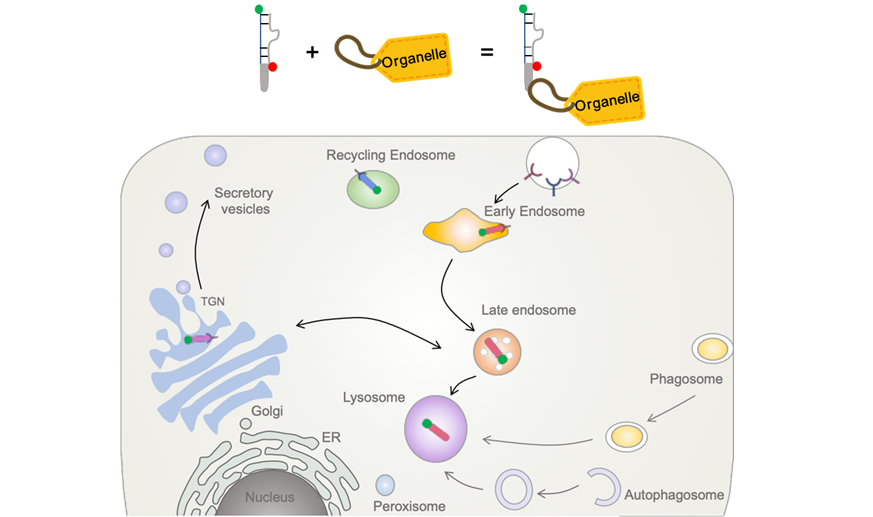
Cell-Specific and Organelle-Specific Targeting
We are also passionate about inventing "targeting modules" to target DNA nanodevices to a designated organelle within a cell, or to a specific cell-type in whole organisms. These are basically trafficking motifs integrated onto DNA nanodevices that engage a cell-surface protein to then traffic DNA reporters to the desired organelle. So far, we can target every kind of endocytic organelle, the trans Golgi network and the phagosome. Along with our chemical sensors, we can now quantitatively image chemicals with accuracies were previously unattainable, in subcellular locations that were previously inaccessible.
Fundamental biology and biomedical applications
Using quantitative chemical imaging, we study how the lumenal chemical mileu of organelles impacts organelle function, which in turn impacts cell function and animal behavior. Examples of our discoveries include the following: showing a new role for lumenal chloride in lysosome function, discovering the first example of a lysosomal Ca2+ importer in the animal kingdom, identifying the precise timescales of membrane-initiated steroid signaling and making the first measures of the membrane potential of several types of intracellular organelles.

